``QUICKIES''
- (a)
- [1992 Xmas]
For an ideal gas the pressure and temperature are directly
related to one of the following velocities of the gas molecules
(underline the correct one): i most probable velocity;
(ii) average velocity; (iii) root-mean-square velocity;
(iv) maximum velocity.
- (b)
- [1994 Final]
A special constant-temperature rocket engine
heats up an ideal gas of ``fuel'' atoms of mass m
to a fixed temperature T
and allows them to escape through a small hole as ``exhaust.''
Which would impart a larger impulse to the
rocket payload - a fuel of hydrogen atoms
or the same total fuel mass of lead atoms?
(Remember, the average kinetic energy of ideal gas atoms
depends only on T and not on m.)
- (c)
- [1995 Final]
For an ideal gas of N particles in thermal equilibrium,
the mean internal energy depends on
[encircle all correct answers]
i temperature
(ii) pressure
(iii) volume
- (d)
- [1996 Final]
In the same room we have a mixture of helium gas, oxygen gas, etc.
What is the ratio of the helium average velocity to that of the oxygen
molecules?
- (e)
- [1997 Final]
For an ideal gas the pressure and temperature
are directly related to one of the following velocities
of the gas molecules: [underline one]
(i) most probable velocity;
(ii) average velocity; (iii) root-mean-square velocity;
(iv) maximum velocity.
[1993 P115 Xmas]
- (a)
- What are the two fixed points on the thermodynamic scale (constant
volume ideal gas thermometer scale)?
- (b)
- How much heat is required to warm 6.0 kg of ice (c=2220 J/kg-K) from
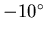 C to
C to 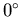 C (staying as ice)?
C (staying as ice)?
- (c)
- How much heat is given off if you freeze 0.3 kg of water initially at
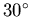 C (c=4190 J/kg-K and L=333,000 J/kg)?
C (c=4190 J/kg-K and L=333,000 J/kg)?
- (d)
- If 0.3 kg of water is dropped onto a 6 kg block of ice
at
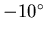 C, what is the final temperature when equilibrium is reached,
and how much ice and/or water is there?
C, what is the final temperature when equilibrium is reached,
and how much ice and/or water is there?
States of Mind and the Heat of Thought [1995 Final]
Consider the following grossly over-simplified model:
A mind is a system 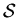 capable of N distinct thoughts,
of which only
capable of N distinct thoughts,
of which only 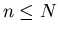 are actually held in memory
at any given time. Any given thought is either
in memory or not; there is no middle ground.
Furthermore, each thought takes the same
amount of ``mental energy"
are actually held in memory
at any given time. Any given thought is either
in memory or not; there is no middle ground.
Furthermore, each thought takes the same
amount of ``mental energy"
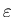 ,
so that a mind with a total available mental energy U
will always have exactly
,
so that a mind with a total available mental energy U
will always have exactly
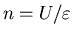 thoughts in memory.
A specific set of n thoughts can be considered one
``fully specified state of mind" for
thoughts in memory.
A specific set of n thoughts can be considered one
``fully specified state of mind" for 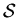 .
We shall make the further (rather insulting) assumption
that every possible fully specified state of mind
with n thoughts is a priori equally likely.
.
We shall make the further (rather insulting) assumption
that every possible fully specified state of mind
with n thoughts is a priori equally likely.
- (a)
- How many different fully specified
states with n thoughts could occupy a mind which has
``mental room" for N thoughts?
- (b)
- What is the entropy
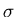 of a mind which has the capacity for 12 thoughts
but currently contains only 4?
of a mind which has the capacity for 12 thoughts
but currently contains only 4?
- (c)
- Explain how to define a mental temperature
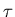 for a given mind, assuming only that you know how
the number of possible fully specified states of that mind
depends on its total mental energy.
[The specific form of said dependence
need not be the one you gave above.]
for a given mind, assuming only that you know how
the number of possible fully specified states of that mind
depends on its total mental energy.
[The specific form of said dependence
need not be the one you gave above.]
- (d)
- If
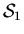 ,
which has very little mental energy U1
but a large mental temperature
,
which has very little mental energy U1
but a large mental temperature 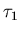 ,
is free to exchange thoughts with
,
is free to exchange thoughts with
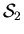 ,
which has enormous mental energy U2
but a small mental temperature
,
which has enormous mental energy U2
but a small mental temperature 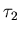 ,
whose mental energy is most likely to increase?
Explain.
(Assume both are isolated mentally from the rest of the world.)
,
whose mental energy is most likely to increase?
Explain.
(Assume both are isolated mentally from the rest of the world.)
- (e)
- Now suppose that one particular mind is in ``mental equilibrium"
with the UBC intellectual community,
which we shall assume has a ``mental temperature"
of
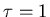 mJ.
If it takes that mind an extra mental energy of
mJ.
If it takes that mind an extra mental energy of
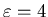 mJ to have a given thought
(in addition to whatever other thoughts it might be having),
what is the probability of that specific thought
being present in that mind at any given time?
mJ to have a given thought
(in addition to whatever other thoughts it might be having),
what is the probability of that specific thought
being present in that mind at any given time?
[1992 P115 Xmas]
A 180 g copper pot contains 200 litre of water at an initial temperature of
20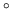 C. A copper rod heated to 900
C. A copper rod heated to 900 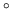 C is dropped into the water.
This causes the water to boil with 5.0 g of water converted to steam. What is
the mass of the copper rod?
C is dropped into the water.
This causes the water to boil with 5.0 g of water converted to steam. What is
the mass of the copper rod?
Specific heat of copper = 386 J/kg.K
Specific heat of water = 4190 J/kg.K
Heat of vaporisation of water = 2256000 J/kg
[1992 P115 Xmas]
An igloo, a hemispherical enclosure built of ice, has an inner radius of
2.5 m . The thickness of the ice is 50 cm. On a cold winter day the outside
temperature is -40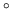 C.
At what rate must thermal energy be generated to maintain the
temperature inside the igloo at
C.
At what rate must thermal energy be generated to maintain the
temperature inside the igloo at 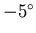 C?
C?
Thermal conductivity of ice = 0.592 W/m
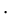
K
A quantity of gas (
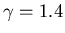 )
expands adiabatically and quasi-statically
from an initial pressure of 2 atm and volume of 2
)
expands adiabatically and quasi-statically
from an initial pressure of 2 atm and volume of 2 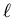 at temperature of
20
at temperature of
20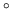 C to twice its original volume.
C to twice its original volume.
- (a)
- What is the final pressure of the gas?
- (b)
- What is the final temperature of the gas?

![]() capable of N distinct thoughts,
of which only
capable of N distinct thoughts,
of which only ![]() are actually held in memory
at any given time. Any given thought is either
in memory or not; there is no middle ground.
Furthermore, each thought takes the same
amount of ``mental energy"
are actually held in memory
at any given time. Any given thought is either
in memory or not; there is no middle ground.
Furthermore, each thought takes the same
amount of ``mental energy"
![]() ,
so that a mind with a total available mental energy U
will always have exactly
,
so that a mind with a total available mental energy U
will always have exactly
![]() thoughts in memory.
A specific set of n thoughts can be considered one
``fully specified state of mind" for
thoughts in memory.
A specific set of n thoughts can be considered one
``fully specified state of mind" for ![]() .
We shall make the further (rather insulting) assumption
that every possible fully specified state of mind
with n thoughts is a priori equally likely.
.
We shall make the further (rather insulting) assumption
that every possible fully specified state of mind
with n thoughts is a priori equally likely.
![]() C. A copper rod heated to 900
C. A copper rod heated to 900 ![]() C is dropped into the water.
This causes the water to boil with 5.0 g of water converted to steam. What is
the mass of the copper rod?
C is dropped into the water.
This causes the water to boil with 5.0 g of water converted to steam. What is
the mass of the copper rod?
![]() C.
At what rate must thermal energy be generated to maintain the
temperature inside the igloo at
C.
At what rate must thermal energy be generated to maintain the
temperature inside the igloo at ![]() C?
C?
![]() )
expands adiabatically and quasi-statically
from an initial pressure of 2 atm and volume of 2
)
expands adiabatically and quasi-statically
from an initial pressure of 2 atm and volume of 2 ![]() at temperature of
20
at temperature of
20![]() C to twice its original volume.
C to twice its original volume.