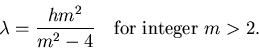
Narrator: ``Faraday has elucidated the nature of electrical phenomena, Maxwell has formulated the physical laws, and Joseph John Thomson has found the ELECTRON. It seems to be a fundamental particle, tied up in the atom. Electrons are discrete pointlike things with negative electric charge, and moving them around creates esoteric phenomena like magnetism, so the atoms that make up everyday stuff must look something like this (holds up a RAISIN BUN) with the mushy brown part embodying most of the mass and the positive charge required to make the overall atom electrically neutral. We just need to nail down the details of how jiggling raisin buns explain everything.''
Balmer and Rydberg [conducting experiments with a Bunsen burner and a nichrome wire]
Balmer scratches head, mumbles:
``This darn high school teaching is driving me crazy,''
and writes on board near H atom spectrum:
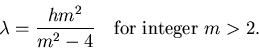
Rydberg:
``Dr.
Balmer,
I decided to visit you in Basel because
I am so very excited with your equation for the H
atom lines. If I turn it upside down I can write it:
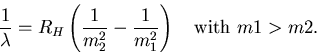
Narrator: ``These empirical formulas are certainly useful. But what do they mean?''
Planck: Enters, takes chalk from Balmer's hand, walks to blackbody spectrum on next blackboard panel and fills in corrected theoretical spectrum.
``I was able to get rid of the so-called
ultraviolet catastrophe and make the theoretical
BLACKBODY SPECTRUM agree with the experimental data
by assuming that the energy of electromagnetic waves
was not a continuous property, but came quantized
in fixed multiples of . . . '' [writes on board]
[Leaves chalk under PHOTOELECTRIC EFFECT.]