Consider a human biceps as shown below.
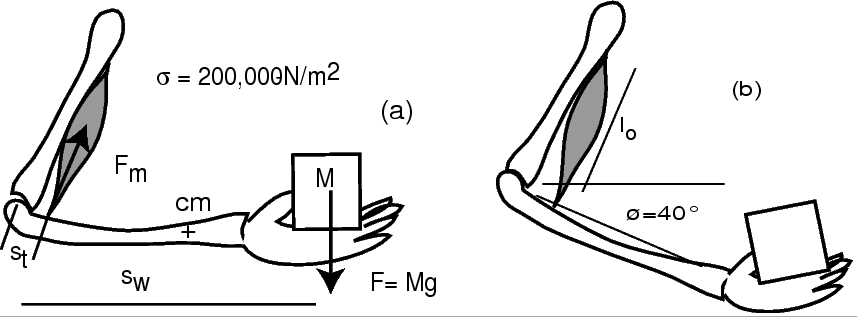
- Measure your biceps length
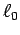 and the distance
and the distance 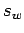 .
Estimate the distance
.
Estimate the distance 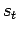 (explain how you do this).
Determine your muscle contraction
(explain how you do this).
Determine your muscle contraction
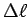 from similar triangles
from similar triangles
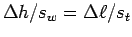 when moving the hand between positions (a) and (b).
Does the maximum contraction agree with the often quoted value
when moving the hand between positions (a) and (b).
Does the maximum contraction agree with the often quoted value
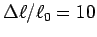 %?1 Determine the average cross sectional area and length
of your biceps muscle to find its volume
%?1 Determine the average cross sectional area and length
of your biceps muscle to find its volume
 and mass
and mass
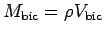 .
What value should you use for the muscle density
.
What value should you use for the muscle density 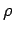 ?
?
- Find a heavy weight, say
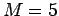 -10 kg,
lift it as quickly as you can
-10 kg,
lift it as quickly as you can 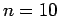 times,2 and record the time
times,2 and record the time
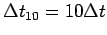 for these 10 cycles.
Estimate the average time
for these 10 cycles.
Estimate the average time
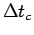 that the muscle spent
contracting in each cycle,
determine your normalized muscle contraction speed
that the muscle spent
contracting in each cycle,
determine your normalized muscle contraction speed
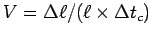 ,
and compare it to the normalized maximum power speed
,
and compare it to the normalized maximum power speed
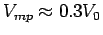 [s
[s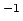 ],
where
],
where 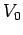 is the intrinsic muscle velocity for humans.3 Comment on the result.
is the intrinsic muscle velocity for humans.3 Comment on the result.
Determine the power needed to raise the weight: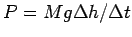 .
Estimate the mass
.
Estimate the mass 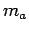 of your forearm
and the approximate position of its center of mass (cm),
so that you can get estimates of the force
of your forearm
and the approximate position of its center of mass (cm),
so that you can get estimates of the force 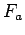 and power
and power 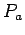 needed to move the forearm.
Add the forces
needed to move the forearm.
Add the forces
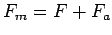 and the powers
and the powers
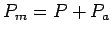 and give estimates of the specific muscle force
and give estimates of the specific muscle force 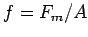 and the specific muscle power of your biceps
and the specific muscle power of your biceps
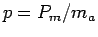 .
.
Make a table of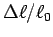 ,
, 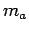 ,
contraction frequency
,
contraction frequency
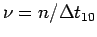 ,
,
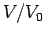 ,
, 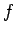 and
and 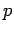 for all team members.
for all team members.
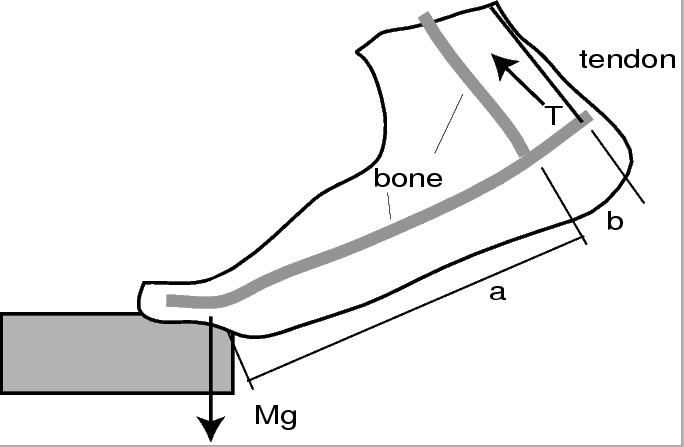
- Calculate the tension
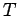 in the Achilles tendon
of each member of your group when he or she stands
on the toes of one foot on a staircase, as shown above.
For that you will have to know the person's body mass
in the Achilles tendon
of each member of your group when he or she stands
on the toes of one foot on a staircase, as shown above.
For that you will have to know the person's body mass 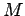 and the relevant dimensions of their foot.
and the relevant dimensions of their foot.
- Measure the radius
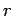 of each person's Achilles tendon and
determine the corresponding specific stress
of each person's Achilles tendon and
determine the corresponding specific stress
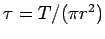 .
.
- Make a table of
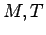 and
and 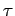 for all team members.
Email this information (in plain text, please!) to Alex
and he will make a compound table of this data for the whole class.
for all team members.
Email this information (in plain text, please!) to Alex
and he will make a compound table of this data for the whole class.
Air is a (mostly diatomic) molecular gas with specific heat
![]() , where
, where ![]() J/mole
J/mole
![]() K
is the gas constant. Recall the Ideal Gas Law
K
is the gas constant. Recall the Ideal Gas Law ![]() ,
where the pressure
,
where the pressure ![]() is in N/m
is in N/m![]() , the volume
, the volume ![]() is in m
is in m![]() ,
,
![]() is the number of moles in the volume
is the number of moles in the volume ![]() and the temperature
and the temperature ![]() is always in
is always in ![]() K.
Differentiate the caloric energy equation,
K.
Differentiate the caloric energy equation,
![]() ,
to get the heat flow rate that must be provided by the lung
to maintain
,
to get the heat flow rate that must be provided by the lung
to maintain ![]() C.
C.
- How many moles are in one liter at this temperature and pressure?
How many moles per second does he inhale?
- How much heat power [Watts] does Robert lose by warming up the air?
- What is the average intake velocity
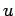 at which the air streams through his nostrils?
at which the air streams through his nostrils?
- Is the flow laminar (
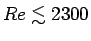 )
or turbulent (
)
or turbulent (
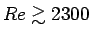 )?4
)?4
- Measure the open area
 of your own nose
and provide this data along with the body mass for each individual.
Plot the area
of your own nose
and provide this data along with the body mass for each individual.
Plot the area  as an allometric relation
as an allometric relation
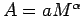 on a log - log graph for the members of your group.
What is the scaling exponent
on a log - log graph for the members of your group.
What is the scaling exponent 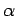 for your data?
What exponent do you expect? Why?
for your data?
What exponent do you expect? Why?