

Next: Amperes
Up: Electrical Units
Previous: Coulombs and Volts
We can also take advantage of the fact that Nature supplies
electric charges in integer multiples of a fixed quantity
of charge17.11
to define some more "natural" units.
For instance, the electric charge of an electron is -e
[where e is the charge of a proton, defined in Eq. (2)].
An ELECTRON VOLT (eV) is the kinetic energy gained
by an electron [or any other particle with the same
size charge] when it is accelerated through a one volt (1 V)
electric potential. Moving a charge of 1 C
through a potential of 1 V takes 1 J of work
(and will produce 1 J of kinetic energy),
so we know immediately from Eq. (2) that
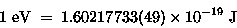 |
(17.17) |
This is not much energy if you are a toaster, but for an electron
(which is an incredibly tiny particle) it is enough to get
it up to a velocity of 419.3828 km/s, which is 0.14% of the speed
of light! Another way of looking at it is to recall that we can
express temperature in energy units using Boltzmann's constant
as a conversion factor. You can easily show for yourself that
1 eV is equivalent to a temperature of 11,604 degrees Kelvin
or about 11,331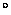 C. So in the microscopic world of electrons
the eV is a pretty convenient (or "natural") unit. But not
in the world of toasters and light bulbs. So let's get back to
"conventional" units.
C. So in the microscopic world of electrons
the eV is a pretty convenient (or "natural") unit. But not
in the world of toasters and light bulbs. So let's get back to
"conventional" units.


Next: Amperes
Up: Electrical Units
Previous: Coulombs and Volts
Jess H. Brewer -
Last modified: Mon Nov 16 17:15:46 PST 2015