

Next: The Magnetic Force
Up: "Direct" Force Laws
Previous: "Direct" Force Laws
First, what is a charge? We don't know!
But then, we don't know what a mass is, either,
except in terms of its behaviour: a mass resists acceleration
by forces and attracts other masses with a gravitational force.
The analogy is apt, in the sense that electrical charges exert
forces on each other in almost exactly the same way as masses do,
except for two minor differences, which I will come to shortly.
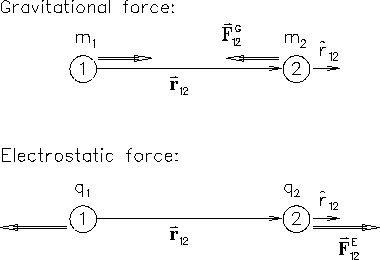
Recall Newton's UNIVERSAL LAW OF GRAVITATION in its most
democratic form: the force
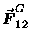 acting on
body #2 (mass m2) due to body #1 (mass m1) is
acting on
body #2 (mass m2) due to body #1 (mass m1) is
where G is the Universal Gravitational Constant,
r12 is the distance between the two masses and
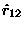 is the unit vector pointing from #1
to #2.
The electrostatic force
is the unit vector pointing from #1
to #2.
The electrostatic force
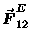 between two charges q1 and q2
is of exactly the same form:
between two charges q1 and q2
is of exactly the same form:
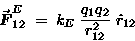 |
(17.1) |
where kE is some constant to make all the units come out right
[allow me to sidestep this can of worms for now!]. Simple, eh?
This force law, also known as the COULOMB FORCE,17.1
has almost the same qualitative earmarks as the force of gravity:
the force is "central" - i.e. it acts along the line joining
the centres of the charges - and drops off as the inverse square
of the distance between them; it is also proportional to each of
the charges involved. [We could think of mass as a sort
of "gravitational charge" in this context.]
So what are the "minor differences?"
Well, the first one is in the sign.
Both "coupling constants" (G and kE)
are defined to be positive; therefore the - sign
in the first equation tells us that the gravitational force
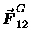 on mass #2 is in the opposite direction
from the unit vector
on mass #2 is in the opposite direction
from the unit vector
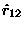 pointing form #1 to #2
-- i.e. the force is attractive, back toward the other body.
All masses attract all other masses gravitationally;
there are (so far as we know) no repulsive forces in gravity.
Another way of putting it would be to say that
"there are no negative masses."
By contrast, electric charges come in
both positive (+) and negative (-) varieties; moreover,
Eq. (1) tells us that the electrical force
pointing form #1 to #2
-- i.e. the force is attractive, back toward the other body.
All masses attract all other masses gravitationally;
there are (so far as we know) no repulsive forces in gravity.
Another way of putting it would be to say that
"there are no negative masses."
By contrast, electric charges come in
both positive (+) and negative (-) varieties; moreover,
Eq. (1) tells us that the electrical force
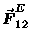 on charge #2 is in the same direction
as
on charge #2 is in the same direction
as
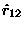 as long as the product q1 q2 is positive - i.e.
as long as the product q1 q2 is positive - i.e.
charges of like sign [both + or both -]
repel
whereas unlike charges attract.
This means that a positive charge and a negative charge
of equal magnitude will get pulled together until their net
charge is zero, whereupon they "neutralize" each other
and cease interacting with all other charges.
To a good approximation, this is just what happens!
Most macroscopic matter is electrically neutral,
meaning that it has the positive and negative charges
pretty much piled on top of each other.17.2
The second "minor difference" between electrical
and gravitational forces is in their magnitudes.
Of course, each depends on the size of
the "coupling constant"
[G for gravity vs. kE for electrostatics]
as well as the sizes of the "sources"
[m1 and m2 for gravity vs. q1 and q2 for electrostatics]
so any discussion of magnitude has to be in reference to
"typical" examples. Let's choose the heaviest stable
elementary particle that has both charge and mass:
the proton, which constitutes the nucleus of a
hydrogen atom.17.3
A proton has a positive charge of
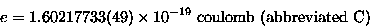 |
(17.2) |
[Don't worry about what a coulomb is just yet.]
and a mass of
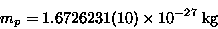 |
(17.3) |
For any separation distance r,
two protons attract each other (gravitationally) with a force
whose magnitude FG is
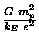 times the magnitude
FE of the (electrostatic) force with which they repel each other.
This ratio has an astonishing value of
times the magnitude
FE of the (electrostatic) force with which they repel each other.
This ratio has an astonishing value of
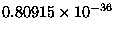 --
the gravitational attraction between the two protons is
roughly a trillion trillion trillion times weaker
than the electrostatic repulsion.
The electrical force wins, hands down. However,
in spite of its phenomenal puniness, gravity can overcome
all other forces if enough mass gets piled up in one place.
This feature will be discussed at length later on, but for now
it is time to discuss the basic magnetic force.
--
the gravitational attraction between the two protons is
roughly a trillion trillion trillion times weaker
than the electrostatic repulsion.
The electrical force wins, hands down. However,
in spite of its phenomenal puniness, gravity can overcome
all other forces if enough mass gets piled up in one place.
This feature will be discussed at length later on, but for now
it is time to discuss the basic magnetic force.


Next: The Magnetic Force
Up: "Direct" Force Laws
Previous: "Direct" Force Laws
Jess H. Brewer -
Last modified: Mon Nov 16 17:01:36 PST 2015
![]() acting on
body #2 (mass m2) due to body #1 (mass m1) is
acting on
body #2 (mass m2) due to body #1 (mass m1) is
![]() on mass #2 is in the opposite direction
from the unit vector
on mass #2 is in the opposite direction
from the unit vector
![]() pointing form #1 to #2
-- i.e. the force is attractive, back toward the other body.
All masses attract all other masses gravitationally;
there are (so far as we know) no repulsive forces in gravity.
Another way of putting it would be to say that
"there are no negative masses."
By contrast, electric charges come in
both positive (+) and negative (-) varieties; moreover,
Eq. (1) tells us that the electrical force
pointing form #1 to #2
-- i.e. the force is attractive, back toward the other body.
All masses attract all other masses gravitationally;
there are (so far as we know) no repulsive forces in gravity.
Another way of putting it would be to say that
"there are no negative masses."
By contrast, electric charges come in
both positive (+) and negative (-) varieties; moreover,
Eq. (1) tells us that the electrical force
![]() on charge #2 is in the same direction
as
on charge #2 is in the same direction
as
![]() as long as the product q1 q2 is positive - i.e.
as long as the product q1 q2 is positive - i.e.