


Next: Resonant formation
Up: Formation of muonic molecules
Previous: Formation of muonic molecules
A straightforward process for muonic molecular formation is via the emission
of an Auger electron:
![\begin{displaymath}
\mu x + YZ \rightarrow [(x\mu y)^{+}ze] + e^{-}.
\end{displaymath}](img79.gif) |
(8) |
The rate for this non-resonant process is typically of the order of 106s-1, hence one muon could catalyze not many more than one fusion,
if this were the only process for formation.
The most recent calculations of the non-resonant molecular formation rates
were performed by Faifman for all the combinations of
collisions [51]. Using the molecular bound state energy levels
and wave functions derived from the improved two level approximation in the
Adiabatic Representation (Section 2.1.3), the rates were calculated
taking into account monopole E0 and dipole E1 Auger transitions to all the bound states (earlier calculations considered only selected
states). Note that the existence of weakly bound states, which is of
paramount importance for resonant formation, also affects non-resonant
formation.
Table 1.2:
Non-resonant muonic molecular formation rates in 106 s-1 by Faifman [51].
| Reaction |
|
Collision energy (cms) [eV] |
| |
|
0.003 |
0.040 |
0.100 |
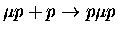 |
|
1.81 |
1.80 |
1.78 |
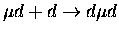 |
|
0.03 |
0.10 |
0.22 |
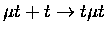 |
|
2.71 |
2.64 |
2.57 |
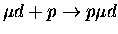 |
|
5.69 |
5.63 |
5.54 |
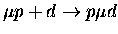 |
|
0.17 |
0.16 |
0.15 |
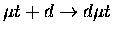 |
|
0.56 |
6.07 |
11.60 |
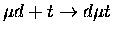 |
|
2.26 |
2.21 |
2.15 |
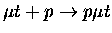 |
|
6.47 |
6.38 |
6.27 |
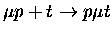 |
|
0.03 |
0.03 |
0.03 |
|



Next: Resonant formation
Up: Formation of muonic molecules
Previous: Formation of muonic molecules