


Next: 6.2.1 Previous measurements
Up: 6 Spin-Peierls system
Previous: .
The first spin Peierls materials were all organic. This may be
attributed to the characteristics of organic materials that (1) the
lattice is soft and its deformation is relatively easy and (2) the
distance between the spin-chains is relatively large, so that they are
magnetically well isolated from each other. As an inorganic
material, CuGeO3 was the first material identified to exhibit a spin
Peierls transition [141].
Figure:
Crystal structure of CuGeO3. In the c-axis direction, CuO2 chains
are present. The lattice parameters are cited from
Ref. [142]; the in-chain magnetic coupling
parameters are from Ref. [143].
 |
The crystal structure of CuGeO3 is shown in
Fig.52. A Cu2+ ion (S=1/2) is
surrounded by six O2- ions, which form a distorted CuO6
octahedron. Cu2+ ions are bridged with four closer O2- ions,
making a CuO2 chain (ribbon) in the c-axis direction. These
chains are supported with Ge4+ ions, which locate at the
tetrahedral site surrounded by four O2- ions. Reflectivity and
photoemission measurements [144] have shown that the
 hybridization of the CuO2 chain is small. Hence the localized-moment
picture of the Cu2+ spins should be good in CuGeO3.
hybridization of the CuO2 chain is small. Hence the localized-moment
picture of the Cu2+ spins should be good in CuGeO3.
In the first report, Hase et al. [141] presented (1) an
isotropic drop of susceptibility at 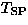 =14 K and (2) the field dependence of
=14 K and (2) the field dependence of  , as signatures of the spin Peierls transition in CuGeO3.
Since then, there have been many experiments performed on this material, and some
of them report typical signatures of spin Peierls transition, as reviewed in the
following.
, as signatures of the spin Peierls transition in CuGeO3.
Since then, there have been many experiments performed on this material, and some
of them report typical signatures of spin Peierls transition, as reviewed in the
following.
-
6.2.1 Previous measurements
-
Superlattice reflections
[#!KamimuraJPSJ94!#,#!PougetPRL94!#,#!HirotaPRL94!#]
-
Softening of the lattice
[#!PoirierPRB95a!#,#!LorenzoPRB94!#,#!HarrisPRB94!#,#!NishiPRB95!#,#!ChenPRB95!#]
-
Structural signature of SP
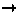 M
M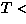 transition
[#!KiryukhinPRB95!#]
transition
[#!KiryukhinPRB95!#]
-
Magnetic phase diagram
[#!HasePRB93!#,#!PoirierPRB95a!#]
-
Spin Peierls gap
[#!NishiPRB94!#,#!FujitaPRL95!#]
-
Spin relaxation measurements
[#!OseroffJAP94!#,#!BrillPRL94!#,#!ItohPRB95!#]
-
.
-
6.2.2 Non-magnetic ion doping
-
6.2.3
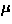 SR measurements
SR measurements
-
Nominally pure CuGeO3
-
Zn-doped systems
-
Si-doped system
-
Discussion
-
Absence of precession in the Zn-doped samples
-
Doping dependence of the size of moment



Next: 6.2.1 Previous measurements
Up: 6 Spin-Peierls system
Previous: .


![]() =14 K and (2) the field dependence of
=14 K and (2) the field dependence of ![]() , as signatures of the spin Peierls transition in CuGeO3.
Since then, there have been many experiments performed on this material, and some
of them report typical signatures of spin Peierls transition, as reviewed in the
following.
, as signatures of the spin Peierls transition in CuGeO3.
Since then, there have been many experiments performed on this material, and some
of them report typical signatures of spin Peierls transition, as reviewed in the
following.